Easier, faster trials, and better results.
Fast, low-admin, audit-proof, connected, secure. iMediTrial's cloud based Clinical Trial Management System innovatively improves data quality and quantity.
Focus on research.
iMediTrial is built to 21 CFR part 11 and GLP standards, on a SOC2 certified architecture and AWS hosting. It handles your data's security, audit and compliance needs so you can focus on research.

Customised, collaboratively.
Efficient trial flow over a cohesive data structure. You don't service your car. Have your trial system customised by a specialist, on a regulatory body friendly data structure.

1
2
3
Benefits:
1
Complexity
iMediTrial's CTMS and EDC+ system handles research and organisational complexity for multiple stakeholders built over a cohesive data structure.
2
Compliant
iMediTrial meets regulatory standards and methodologies including ALCOA+, GLP, and FDA (21 CFR Part 11). It's built on a secure SOC2 compliant architecture and AWS hosting.
3
Cost effective
An Australian company, with a background in control systems engineering, developed a beautiful dashboard interface that is a pleasure to use. Let us show you how it can improve data quality and quantity, and reduce admin.


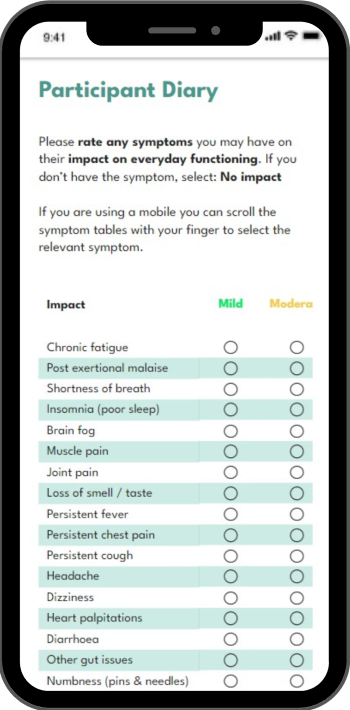
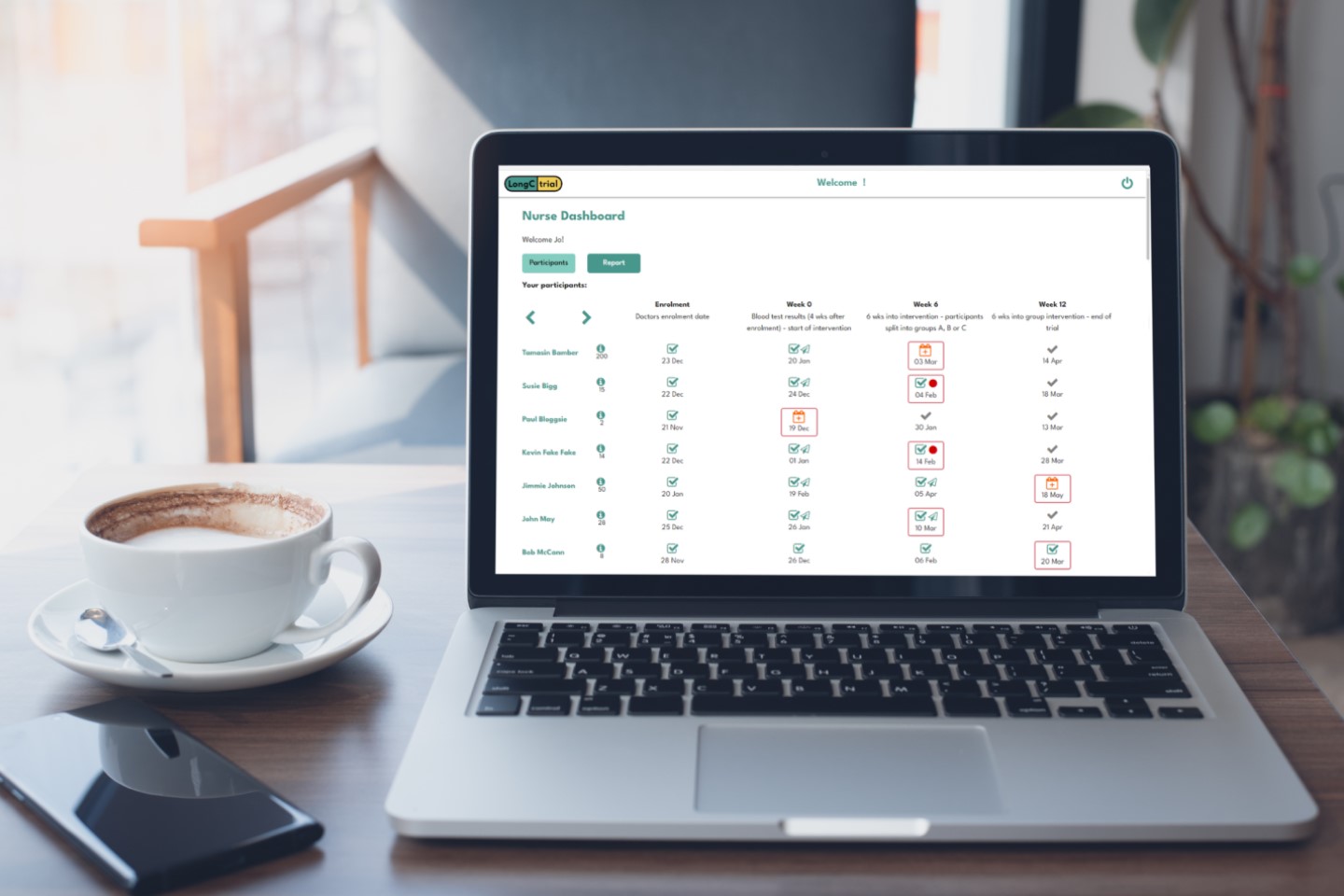
Our goal is to make your trial run so efficiently it almost runs itself.
We want to simplify research and ensure accuracy. iMediTrial automates admin and audit trails, allowing you to focus on research.
Our story
The COVID pandemic was a once in a lifetime event that those of us who lived through it will never forget.
iMediTrial was built in the height of the pandemic, when an innovative, Australia wide, telehealth based trial needed a solution to a difficult problem. A team consisting of doctors, nurses, pharmacy and logistics had to have a randomised intervention and measurement device in the hands of participants in the first 5 days of contracting the virus.
A multi-disciplinary team that included a former McLaren vehicle dynamics engineer and a laparoscopic surgeon took on the challenge.
The trial ran successfully over 20 months with 275 participants Australia wide.
iMediTrial was born.
iMediTrial was built in the height of the pandemic, when an innovative, Australia wide, telehealth based trial needed a solution to a difficult problem. A team consisting of doctors, nurses, pharmacy and logistics had to have a randomised intervention and measurement device in the hands of participants in the first 5 days of contracting the virus.
A multi-disciplinary team that included a former McLaren vehicle dynamics engineer and a laparoscopic surgeon took on the challenge.
The trial ran successfully over 20 months with 275 participants Australia wide.
iMediTrial was born.
What we offer
Intuitive Design
Design so simple that the trial flow explains itself, and so beautiful it's a pleasure to use.
Device friendly
Take your participant details and track their symptoms on the run with a web browser or the option of a native mobile app.
Engineering Support
Speak to the engineers who are building your solution. Our hands on team guides you through every step of the way.
Security
iMediTrial’s security is backed by a SOC2 and GDPR compliant framework and AWS hosting - the hosting used by the ATO, CSIRO, CBA and Telstra.
Testimonials
"Our trial was time-critical. The intervention package had to be sent within hours of the participant's registration. iMediTrial allowed us to vet eligibility, generate doctors scripts, manage logistics and then monitor critical participants in one place.
We couldn't have run our trial safely without it"
Principal Investigator, PACtrial
"It saved us hundreds of thousands on trial management and admin personnel. More than that, we were able to start-up a lean, care-focussed trial in hardly any time - from scratch."

Chairman, Lumina Medical Research
"Perfect for trial admin. The ability to manage expressions of interest, registration, consent, doctors enrolment and then monitor the participants trial progress in one place was very useful."

Research Director, NIIM
"It's a pleasure to work with. Very user friendly. It kept track of everything and made it easy to delegate when I was on holidays."

Research Nurse, Long COVID Trial
We love working with people solving complex problems
We’d love to help make your research a success. Get in touch.
